Name: Helium: Symbol: He Atomic Number: 2: Atomic Mass: 4.002602 atomic mass unit: Number of Protons: 2: Number of Neutrons: 2: Number of Electrons: 2: Melting Point. According to Bohr, as per the energy level, the filling of electrons in each shell will be 2, 8, 8, 18, 32. The atomic number given are 2, 10, 18, 36, 54, 86 which shows that these elements have completely filled orbitals and are noble gases or zero group members. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that.
The elements of the periodic table sorted by atomic number
click on any elements name for further chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Atomic number | Name chemical element | Symbol |
| - Name alphabetically | 1 | Hydrogen | H |
| - Atomic number | 2 | Helium | He |
| - Symbol | 3 | Lithium | Li |
| - Atomic Mass | 4 | Beryllium | Be |
| - Electronegativity | 5 | Boron | B |
| - Density | 6 | Carbon | C |
| - Melting point | 7 | Nitrogen | N |
| - Boiling point | 8 | Oxygen | O |
| - Vanderwaals radius | 9 | Fluorine | F |
| - Year of discovery | 10 | Neon | Ne |
| - Inventor surname | 11 | Sodium | Na |
| - Elements in earthcrust | 12 | Magnesium | Mg |
| - Elements in human body | 13 | Aluminum | Al |
| - Covalenz radius | 14 | Silicon | Si |
| - Ionization energy | 15 | Phosphorus | P |
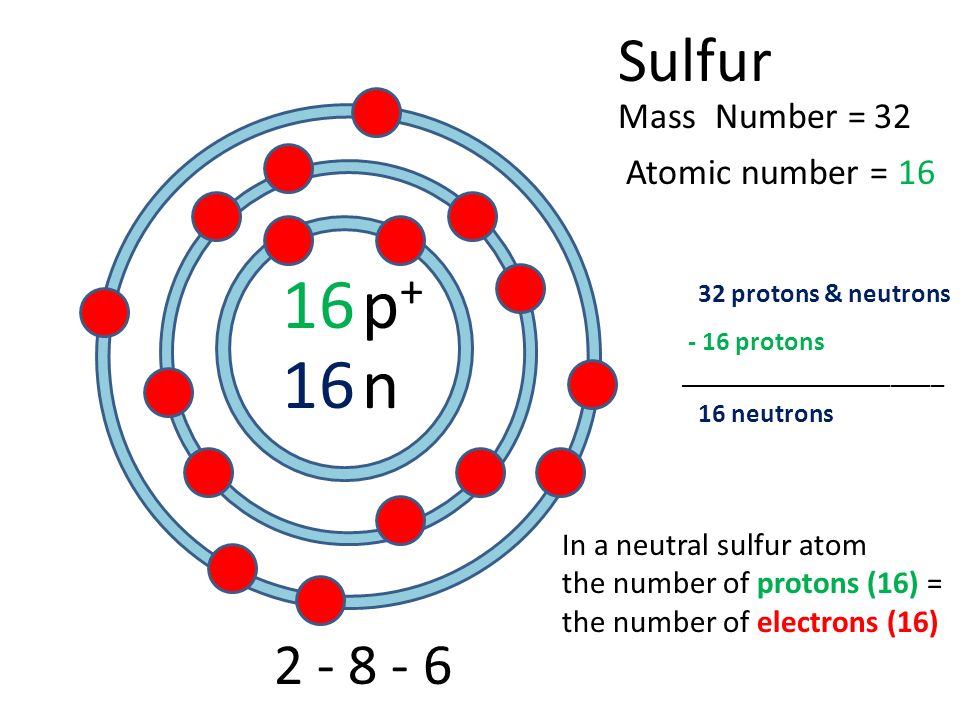
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element is Hydrogen and the last is Ununoctium. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 16 | Sulfur | S |
| 17 | Chlorine | Cl | |
| 18 | Argon | Ar | |
| 19 | Potassium | K | |
| 20 | Calcium | Ca | |
| 21 | Scandium | Sc | |
| 22 | Titanium | Ti | |
| 23 | Vanadium | V | |
| 24 | Chromium | Cr | |
| 25 | Manganese | Mn | |
| 26 | Iron | Fe | |
| 27 | Cobalt | Co | |
| 28 | Nickel | Ni | |
| 29 | Copper | Cu | |
| 30 | Zinc | Zn | |
| 31 | Gallium | Ga | |
| 32 | Germanium | Ge | |
| 33 | Arsenic | As | |
| 34 | Selenium | Se | |
| 35 | Bromine | Br | |
| 36 | Krypton | Kr | |
| 37 | Rubidium | Rb | |
| 38 | Strontium | Sr | |
| 39 | Yttrium | Y | |
| 40 | Zirconium | Zr | |
| 41 | Niobium | Nb | |
| 42 | Molybdenum | Mo | |
| 43 | Technetium | Tc | |
| 44 | Ruthenium | Ru | |
| 45 | Rhodium | Rh | |
| 46 | Palladium | Pd | |
| 47 | Silver | Ag | |
| 48 | Cadmium | Cd | |
| 49 | Indium | In | |
| 50 | Tin | Sn | |
| 51 | Antimony | Sb | |
| 52 | Tellurium | Te | |
| 53 | Iodine | I | |
| 54 | Xenon | Xe | |
| 55 | Cesium | Cs | |
| 56 | Barium | Ba | |
| 57 | Lanthanum | La | |
| 58 | Cerium | Ce | |
| 59 | Praseodymium | Pr | |
| 60 | Neodymium | Nd | |
| 61 | Promethium | Pm | |
| 62 | Samarium | Sm | |
| 63 | Europium | Eu | |
| 64 | Gadolinium | Gd | |
| 65 | Terbium | Tb | |
| 66 | Dysprosium | Dy | |
| 67 | Holmium | Ho | |
| 68 | Erbium | Er | |
| 69 | Thulium | Tm | |
| 70 | Ytterbium | Yb | |
| 71 | Lutetium | Lu | |
| 72 | Hafnium | Hf | |
| 73 | Tantalum | Ta | |
| 74 | Tungsten | W | |
| 75 | Rhenium | Re | |
| 76 | Osmium | Os | |
| 77 | Iridium | Ir | |
| 78 | Platinum | Pt | |
| 79 | Gold | Au | |
| 80 | Mercury | Hg | |
| 81 | Thallium | Tl | |
| 82 | Lead | Pb | |
| 83 | Bismuth | Bi | |
| 84 | Polonium | Po | |
| 85 | Astatine | At | |
| 86 | Radon | Rn | |
| 87 | Francium | Fr | |
| 88 | Radium | Ra | |
| 89 | Actinium | Ac | |
| 90 | Thorium | Th | |
| 91 | Protactinium | Pa | |
| 92 | Uranium | U | |
| 93 | Neptunium | Np | |
| 94 | Plutonium | Pu | |
| 95 | Americium | Am | |
| 96 | Curium | Cm | |
| 97 | Berkelium | Bk | |
| 98 | Californium | Cf | |
| 99 | Einsteinium | Es | |
| 100 | Fermium | Fm | |
| 101 | Mendelevium | Md | |
| 102 | Nobelium | No | |
| 103 | Lawrencium | Lr | |
| 104 | Rutherfordium | Rf | |
| 105 | Dubnium | Db | |
| 106 | Seaborgium | Sg | |
| 107 | Bohrium | Bh | |
| 108 | Hassium | Hs | |
| 109 | Meitnerium | Mt | |
| 110 | Darmstadtium | Ds | |
| 111 | Roentgenium | Rg | |
| 112 | Copernicium | Cn | |
| 113 | Nihonium | Nh | |
| 114 | Flerovium | Fl | |
| 115 | Moscovium | Mc | |
| 116 | Livermorium | Lv | |
| 117 | Tennessine | Ts | |
| 118 | Oganesson | Og |
Click here: for a schematic overview of the periodic table of elements in chart form
Do you need to know the weight of some molecules? Try our Molecular Weight Calculator!
Atomic Number 22
Please report any accidental mistake in the above statistics on chemical elements
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Atomic Number 24
/GettyImages-1143649584-04e88f5111ab4417bc8d8e3fe54566ef.jpg)
Lenntech DMCC (Middle East)

Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copyright © 1998-2021 Lenntech B.V. All rights reserved




